Curriculum 'User Guide Viedoc 4'
Using Reference Data Download PDF
1 Reference Data
- It is possible to have a centralized reference data for different data sources, that makes it possible to fill in, for a certain study, the reference data coming from different data sources that would be automatically populated to the subject forms, instead of having to open that respective form to enter the reference data manually for each subject.
- This is done by configuring the reference data as described in the following sections, in Viedoc Designer, Admin and Clinic.
2 Terminology
- Reference data scope - a set of measurements and the parameters that might affect the respective reference data ranges, that are going to be used in the same lab data form. One or more reference data scopes can be configured in Viedoc Designer > Global Settings, as set(s) of variables and factors (see definitions below).
- Reference data source - a source that provides reference data (e.g. a lab).
The source is defined and linked to one or more reference data sources in order to define which measurements the source carries out, which are the parameters that might affect the results, as well as the specific ranges/units.
The source is also linked to one or multiple sites in the study.
Reference data sources are managed in Viedoc Admin by users having the role Reference data source manager. - Factor - a parameter which affects the reference data, e.g. a subject’s gender - may affect the normal range for a test result.
- Variable - A variable is typically a specific measurement to be carried out.
- Target type - Item of a certain type of information that a source (lab) can provide (such as range, unit, low/high values, etc.) for a specific measurement (defined by a variable). Any number of target types can be defined by the user.
3 Reference data in Viedoc Designer
- Design your study including the form(s) that are going to use the reference data (e.g. lab data form).
- Define the reference data scope(s) in the Global Settings. There is a section called Reference data scopes that provides information on the number of scopes defined, when this section was last edited and by who. By clicking the edit button next to it a new page displays listing all the existing reference data scopes by their scope name. Here you can choose to edit an existing scope or to add a new one. Deletion of scopes is possible until it is used in Viedoc Clinic.
- For a reference data scope, you can enter/edit the following: • Scope name • Factors • Variables
- Set the permissions for the users that are going to be allowed to perform operations on reference data in Viedoc Clinic. There are three permission levels that can be set: • View reference data • Edit reference data • Publish reference data
- Complete instructions on how to configure all the above can be found in Reference Data in Viedoc Designer
- Note! The global design settings have to be published and assigned to your study in order for the reference data settings to be effective and available in Viedoc Admin and in Viedoc Clinic.
4 Reference data in Viedoc Admin
- Assign the Reference data source manager role as a System Role to the user(s) that are supposed to be working with the reference data sources in Viedoc Admin.
- In Viedoc Admin you manage the Reference data sources and perform the mapping between the reference data source and the reference data scope(s). One data source can be linked to one or many data scopes.
- Complete instructions on how to configure all the above can be found in Reference Data in Viedoc Admin
5 Reference data in Viedoc Clinic
- On the landing page, clicking the reference data button will display all the data source - data scope combinations defined for the sites you have access to in your study.
- A Viedoc Clinic user can access and work with the reference data editor according to the permissions assigned to the respective role in Viedoc Designer (see Reference Data in Viedoc Designer):
- The user must have at least View reference data permission for the respective role in order to be able to view the reference data defined for the respective study.
- If the user role has the Edit reference data permission, then it is possible to open each of the reference data sets, edit and save the changes.
- If the user role has the Publish reference data permission, then it is possible to publish the reference data previously entered and saved.
- Once the configured reference data set is published, this becomes available in the subject form that was designed to use the respective reference data.
- Complete instructions on how to manage the reference data in Viedoc Clinic can be found in Reference Data in Viedoc Clinic
6 Reference data use case
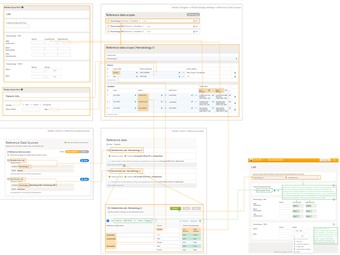
- This section illustrates an example of using reference data in Viedoc, from the settings in Viedoc Admin and Viedoc Designer to the values published in Viedoc Clinic and automatically populated in the respective form.
- Make sure that you have the user roles and permissions correctly defined for being able to work with reference data.
- Identify/design the form(s) that will be used to be automatically populated with the reference data values. In the example shown in the image, this is the Lab form, and the items that could be regarded as reference values are the Low Normal, High Normal and Range.
- Consider the provider of those reference data values and the factors that affect the provided values (such as gender or age). Define in Viedoc Designer, accordingly, one or more Referene data scope(s). In our example, a reference data scope was defined for Hematology U with:
- Gender and Age as Factors - because these are the factors that the respective reference values might depend on.
- Three Variables defined, one for each of the following measurements: Leukocytes, Lymphocytes and Neutrophils. For each variable, there are two target types defined, corresponding to the Low Normal and High Normal fields in the form.
- After finalizing the design of reference data scope(s), publish the Global design settings, so that the defined reference data scope(s) will become available in Viedoc Admin and Viedoc Clinic.
- In Viedoc Admin, go to Reference data source(s) and define the providers of the reference data values. Link each of them to the respective scope(s) and site(s). In our example we have defined two reference data sources, each of them linked to the Hematology U scope, one for the Uppsala site and one for the Stockholm site.
For each of the defined source-scope combinations, reference data values sets will become available in Viedoc Clinic. - In Viedoc Clinic, on the landing page, click the Reference data icon. The list with all the reference data source - scope combinations will be displayed. Click Open reference data editor to enter the reference data values. In our example, we enter the values for the Akademiska Lab, Hematology U. First, select the time period the values are valid for, then add your factors (Gender in this case) and enter the values.
After having filled-in all the data, Save and then Publish.... It is only after publishing when the data will become available for the forms to be filled-in. - Open the respective form (Lab in our case). Viedoc automatically identifies those forms which have items that belong to Reference data scopes and display a section where scopes can be linked to sources
• Link the scope(s) (Hematology U in this case) to a reference data source (we chose Akademiska Lab). If you don't make any selection for the data source, then no values will be automatically filled-in, they will be editable so that the values can be entered manually.
• Set the Collection date and time
It is checked how the dates were set for the variables in the scope in Viedoc Designer (in this case the dates were set to EventDate, respective LAB_DATE) and it is verified if the respective dates are included in the time period that defines the reference values for that source-scope combination. If so (which is the case in our example), the respective values are populated in the form depending on the defined factors (gender in our example, male for the shown form, so the values defined for male will be used.) - For the Mono and Baso, in the bottom of the form, since no scope was defined for those, they remain empty and editable, so that the respective values can be entered manually.
- All the detailed instructions on how to perform the operations related to reference data can be found at: