Curriculum 'User Guide Viedoc 4'
Deleting a study Download PDF
1 Introduction
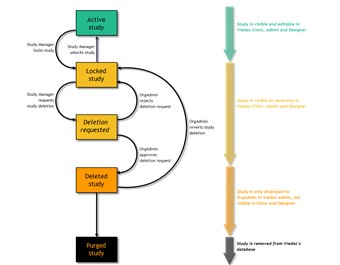
- A study can be permanently deleted from Viedoc when the study is locked. Deletion is initiated by the Study Manager, who can submit a request to delete the study from Viedoc to the Organization Administrator. The Organization Administrator then can approve or reject a request for study deletion.
- After study deletion is requested by the Study Manager and approved by the Organization Administrator, the study is shelved on Viedoc's database. A deleted study is not visible in Viedoc Clinic or Viedoc Designer, the study is only displayed in the study overview page of the Organization Administrator in Viedoc Admin.
- The Organization Administrator is able to revert the deletion of a study within 180 days after the deletion request has been approved. After this period, the study will be permanently purged from Viedoc's database, and all study details and data will be permanently removed. It will not be possible to find any traces of the study and the subjects included.
- For traceability, all study delete actions are audit trailed. You can download a report that provides a full history of all requests for study deletion, approvals of study deletion, and reversions of study deletion that are performed in the study, including who performed the actions and when (date and time in UTC), and the reason that was given for deleting the study or reverting study delete.
2 Requesting study deletion - STM
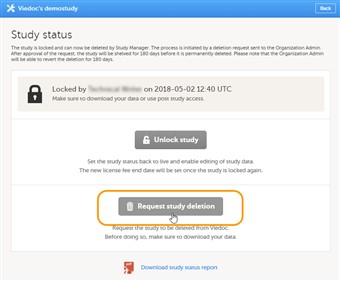
- A request for study deletion can only be submitted by the Study Manager. A study can only be deleted after the study is locked. For information on how to lock a study, see Lock study.
- Note! Before deleting the study, make sure that you have downloaded the user report, the data export archive and the study design. Download and archive the study data in the formats necessary, available formats are Excel, CSV, ODM and PDF. For instructions, see: 1. Downloading the user report in User Management (Viedoc Admin) . 2. Data export (Viedoc Clinic). 3. Export a design (Viedoc Designer).
- To submit a request for study deletion, follow the steps below.
1. Open the study in Viedoc Admin and click Study settings.The Study settings dialog opens. 2. Click the blue pen icon.The study status pop-up opens. 3. Click Request study deletion.The request study delete pop-up opens. 4. Select whether the following actions are done or not done: • Download user report • Download the data export archive required • Download the study design. Enter a reason for deleting the study, and enter your password. 5. Click Request study deletion. The Study status page displays that deletion of the study is requested, by whom and when (date and time in UTC), and the study delete request will be sent to the Organization Administrator. All Study Managers of the study and Organization Administrators will be notified of the request by email. - When study deletion has been requested, the study page will display the status 'Study delete requested by ... on ...' Until the request has been approved, the study will be in locked state and visible in Viedoc Clinic and Viedoc Designer.
3 Approving a study delete request - OA
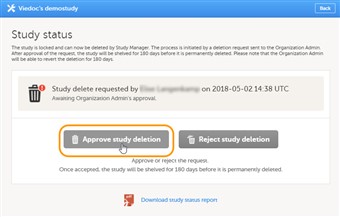
- Note! Before approving the deletion of a study, make sure that the necessary user reports, data export archive and study design are downloaded.
- To approve a request for study deletion, follow the steps below.
1. Open the study in Viedoc Admin and click Study settings. The Study settings dialog opens. 2. Click the blue pen icon to the right of the respective study deletion request. The study status pop-up opens. 3. Click Approve study deletion. A pop-up opens, listing whether the following actions are done or not done by the study manager: • Download user report • Download the data export archive required • Download study design 4. If you agree that all necessary actions are completed, enter a reason for approval of study deletion, and enter your password. 5. Click Approve study deletion. The study status pop-up displays that the study deletion request is confirmed, by whom, and when (date and time in UTC). All Study Managers and Organization Administrators will be notified of the approval by email. - When study deletion is approved, the study will not be visible anymore in Viedoc Clinic or Viedoc Designer, and all user roles will be inactivated. The study will only be displayed to the Organization Administrator in the study overview page.
4 Rejecting a study delete request - OA
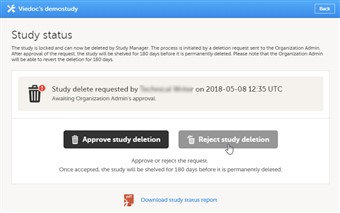
- To reject a request for study deletion, follow the steps below.
1. Open the study in Viedoc Admin and click Study settings. The Study settings dialog opens. 2. Click the blue pen icon to the right of the respective study deletion request. The study status pop-up opens. 3. Click Reject study deletion. A pop-up opens. 4. Enter a reason for rejecting study deletion, and enter your password. 5. Click Reject study deletion. All Study Managers and Organization Administrators will be notified of the rejection by email.
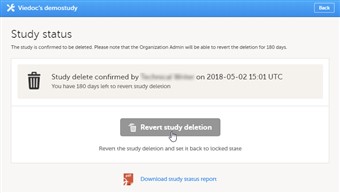
5 Reverting study deletion - OA
- Note! Deletion of a study can be reverted within 180 days after study deletion was approved. The study will then be set back to locked state.
- To revert study deletion, follow the steps below.
1. Open the study in Viedoc Admin. The study status pop-up opens. 2. Click Revert study deletion. A pop-up opens 3. Enter a reason for reverting study deletion, and enter your password. 4. Click Revert study deletion. All Study Managers and Organization Administrators will be notified of the reversion of study deletion by email. The study will be set back to locked state and be visible again in Viedoc Clinic and Viedoc Designer.
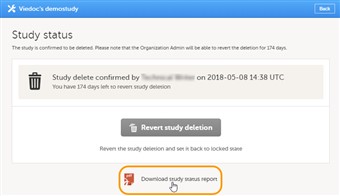
6 Downloading the study status report
- To download the study status report, follow the steps below.
1. Open the study in Viedoc Admin. The Study status dialog opens. 2. Click Download study status report. A PDF is downloaded that lists all database lock and delete actions, including when and by whom the study was locked/deleted, and the reason that was given for locking/deleting the study.