Curriculum 'User Guide Viedoc 4'
Signing of data Download PDF
1 Signature
- Data is signed by the investigator. Signing can be done on form, visit or study level through the signing console.
- Click the icon located in the icons section on the top right side of the page when you want to access the signing console.
2 Signing console
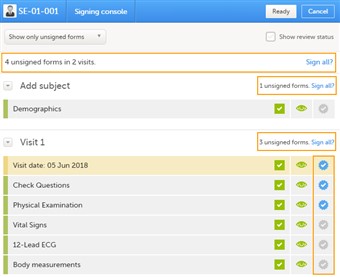
- The signing console helps the investigator to efficiently and easily access and sign-off all unsigned forms. Forms that previously have not been accessed by the investigator do not have the eye icon highlighted.
- To review a form, simply click the form bar. When the form is closed, you will end up in the signing console again.
- To see if data has been reviewed by the CRA or DM, check the "show review status" check box.
- To mark a form as signed, simply check the signing check box to the right. Please observe that data can be signed individually by form, by entire visits or study by clicking the appropriate Sign all link.
3 Confirm signing
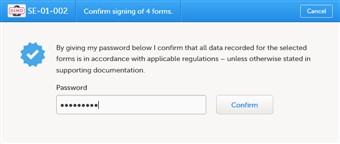
- Whenever you are ready to sign, click the "ready" icon located in the top bar of the page.
- Enter your password and click confirm.
- The text explains the default meaning of the signature in Viedoc when the investigator signs data. It is a generic text meant to cover all the regulations under which any study is conducted. The regulations are different according to the study, and it is the responsibility of the Study manager/Study Coordinator (or any responsible for the study site) to inform the Investigator about the regulations.
4 Signature definition
- As a Viedoc user, you are aware of that all electronic signatures created in the system are intended to be the legally binding equivalent of a traditional handwritten signature.
- In Viedoc the purpose/meaning of a signature is always “responsibility” as used in Sec. 11.50 of FDA 21 CFR part 11. The signer is thereby acknowledging his/her responsibility for the entered data. Viedoc keeps account of what was signed, who signed it and when the signature was performed.
5 Video tutorial
- Signing data - Click link to play video