Curriculum 'User Guide Viedoc 4'
System overview Download PDF
1 System architecture
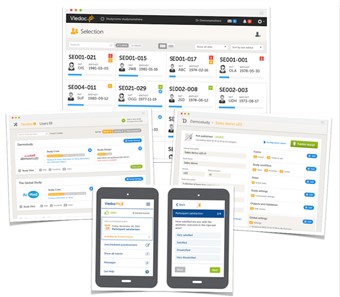
- The Viedoc platform consists of four different applications;
• Viedoc Clinic - for site staff and project team members that need to have access to CRF data
• Viedoc Admin - for parts of the study team to handle users, sites and updates in study designs
• Viedoc Designer - for the study developer to design the study
• ViedocMe - for the subjects to submit questionnaire/diary data to the eCRF
2 System environment
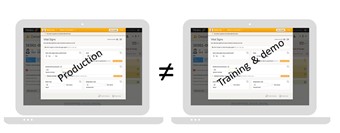
- Your organization has been provided with two separate environments/server areas: one for training/demo studies and one for production studies. Please observe that a production study can be set to operate in training mode, which is different from a training study. A training study will never be taken into production and thus should always be located in the training/demo environment. Contact your organization administrator to get access to the respective area.
- Good to know - Studies and/or study designs are easily transferred from one environment to the other via the CDISC ODM export and import feature.
- Important! We (PCG Solutions AB) do not guarantee that studies running on the training environment are completely and continuously backed-up. The training environment should never be used for a production study.
3 System languages
- Viedoc Clinic is available in the following languages: • English • Chinese (Simplified) • German • Japanese • Polish • Spanish • French • Swedish
- Viedoc Admin and Viedoc Designer are available in the following languages: • English • Chinese (Simplified) • Japanese • Swedish
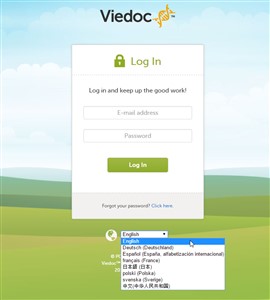
- You can select the language on the Viedoc login window, before logging in to Viedoc (see image).
- You can also change the language settings once you are logged in. To change the language, follow the steps below:
1. Click the settings button (wheel) in the top right corner of the window, and select Edit your profile. The user settings pop-up opens. 2. In the field System language, select the language of your choice from the drop-down menu. 3. Click Save changes to save the changes. The user settings pop-up closes, and the system language changes to the language of your choice. - ViedocMe is available in the following languages:
• Arabic • Bulgarian • Czech • German • Greek • Spanish • Finnish • French • Hungarian • Italian • Japanese • Kazakh • Norwegian (Bokmål and Nynorsk) • Dutch • Polish • Portuguese • Russian • Slovak • Serbian • Swedish • Thai (Thailand) • Turkish • Ukrainian • Chinese (Simplified) • Malay - Would you require any additional language that is not listed above, please contact us at PCG Solutions.
4 Organization
- By default, every organization has one appointed organization administrator. This person has been trained by PCG Solutions and is responsible for providing access to users within the own organization and for adding new studies to the platform. The organization administrator can invite more people to become administrators for the organization.
- Important! It is the responsibility of the organization administrator to make sure that all users within the organization have received appropriate training for their respective tasks.
5 Licensing
- All production studies need to have a valid license before they can be taken into production. The license is provided by PCG Solutions AB. The license fee is based on several factors such as phase of the study and duration of the study. The license fee is charged as long as the study is ongoing, which means, until the study is locked in Viedoc.
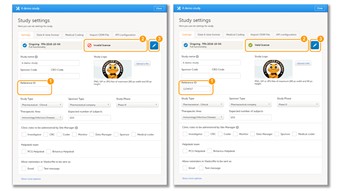
- Every license is connected to a reference ID. The reference ID can be found on the signed study work order (WO), and should be entered in the field Reference ID in the Study settings in Viedoc Admin (nr. 1 in the image). Upon entry of the reference ID, the reference ID is verified. If the reference ID is valid, the text "Valid license key" will be indicated at the following places: • Study settings in Viedoc Admin (nr. 2 in the image), • Studies list in Viedoc Admin, • Study status in Viedoc Admin (nr. 3 in the image). Once the reference ID has been verified, the study can be taken into production. A study is in production mode once the first site is set to production. After the study is set to production mode, the Reference ID is being locked and there is no way to unlock it afterwards.
- For more information regarding license fee and reference ID, please contact PCG Solutions AB.
6 Keep yourself updated
- Viedoc is being developed in a rapid pace. To make sure you are using the platform correctly and to its full potential, use this guide as a refresher after every new release.
- Brief information about new and updated functionality after every release can be found in: • the Release notes, which are sent out before every release, and can be downloaded from the Viedoc website, click here (International website) or here (Japanese website). • the eLearning, in the section "What's new in the latest release".
- More detailed information about the updated functionality is available in the eLearning. Click here for an overview of the latest updated and new sections in the eLearning.
7 Support
- Viedoc enables you to easily setup your own first line support in your production studies. For additional training and/or questions regarding the use of the platform in general, please contact the PCG Solutions representative appointed to your organization.
8 Audit, compliance and other regulatory aspects
- Viedoc fulfils all relevant requirements in the industry. For more detailed information regarding system compliance please do not hesitate to contact us.
- We welcome all organizations to audit us. In fact, we love audits. An audit can either be a physical visit to any of our offices or that you ask us to complete a questionnaire with your questions. We even have an independent audit report that you can purchase for a smaller fee. The choice is yours, we are flexible.