Curriculum 'User Guide Viedoc 4'
Duplicate a design Download PDF
1 Duplicate design
- This is used to either create a new version by copying an existing version, or revise an existing version.
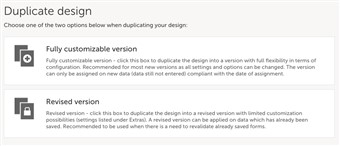
2 Version and revision
- “Fully customizable version” creates a new version, e.g. if we are currently in version 1.4, we will make a copy of that and get version 2.0. “Revised version” will also make a copy but instead create a revision with version 1.5.
Please note: If the “Revised version” option is not available in the Designer even though it is the latest revision, it can be one of two reasons:
• This revision has not yet been assigned or applied to any site. We should then continue working with the existing revision and the way forward is then to “Unpublish” (see lesson "Publish") the design, and then “Unlock” it. When this is done we can continue working with the revised version.
• The Designer-application has not yet picked up that the revision has been assigned or applied. Then please refresh the page and the situation should resolve.
3 Create new version or revise existing version?
- In a perfect world where the version of the study design that is assigned at study start is perfect, new versions should only be used when there is an actual need to have different versions of the “study” over time or on different sites, i.e. due to protocol amendments, or other legit differences like data collection restrictions in different countries.
The general rule is therefore:
• Additional settings are made to correct an error or to complete an incomplete setup, and is applicable to already entered data → Revise current study design version(s)
• Additional settings are made to create a new study design track, and is not applicable for existing data → Create new study design version
However, some changes are not allowed as part of a revision and requires creation of new versions more often than the rule suggests. - The following settings can be revised as part of a revision of a study design version:
• Forms *
• Study workflow *
• Output and Validation
* With the restriction that the study design ID:s (events, activities, forms, item groups and items), item dictionary (“choice”) codes and any items involved in randomization cannot be changed.
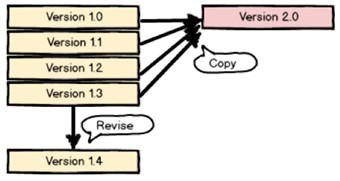
Only the latest revision of each version can be used as starting point of a revision. If version 1 has been revised three times, we have versions 1.0, 1.1, 1.2 and 1.3. Then only version 1.3 can be used as starting point for the fourth revision, 1.4.
All versions can be copied to create a new version. If we only have one version with three revisions, we can copy any of the versions 1.0, 1.1, 1.2 and 1.3 to create version 2.0.
4 Consequences for SDV
- In Viedoc Clinic, the SDV breaks when a new version or revision of the study design is applied, so that the review status reflects which data were affected by the design change and thus should be reviewed again.
- The SDV for individual items breaks when: • the measurement unit is changed, • the field label is changed, or • the options are changed.
- The SDV on all items within an item group breaks when: • the group label is changed, • the static text in a group is changed, or • the order of items is changed.